Avance Biosciences™ is a CRO specialized in QPCR assay development supporting researchers around the world. We offer a broad range of QPCR Assay Development services, including assay design, assay validation, sample testing, and technology transfer under GLP compliance to support drug research and development. Avance Biosciences™ has the technical expertise, GLP regulatory compliance, fast turn-around time, and outstanding customer service to ensure the success of your project.
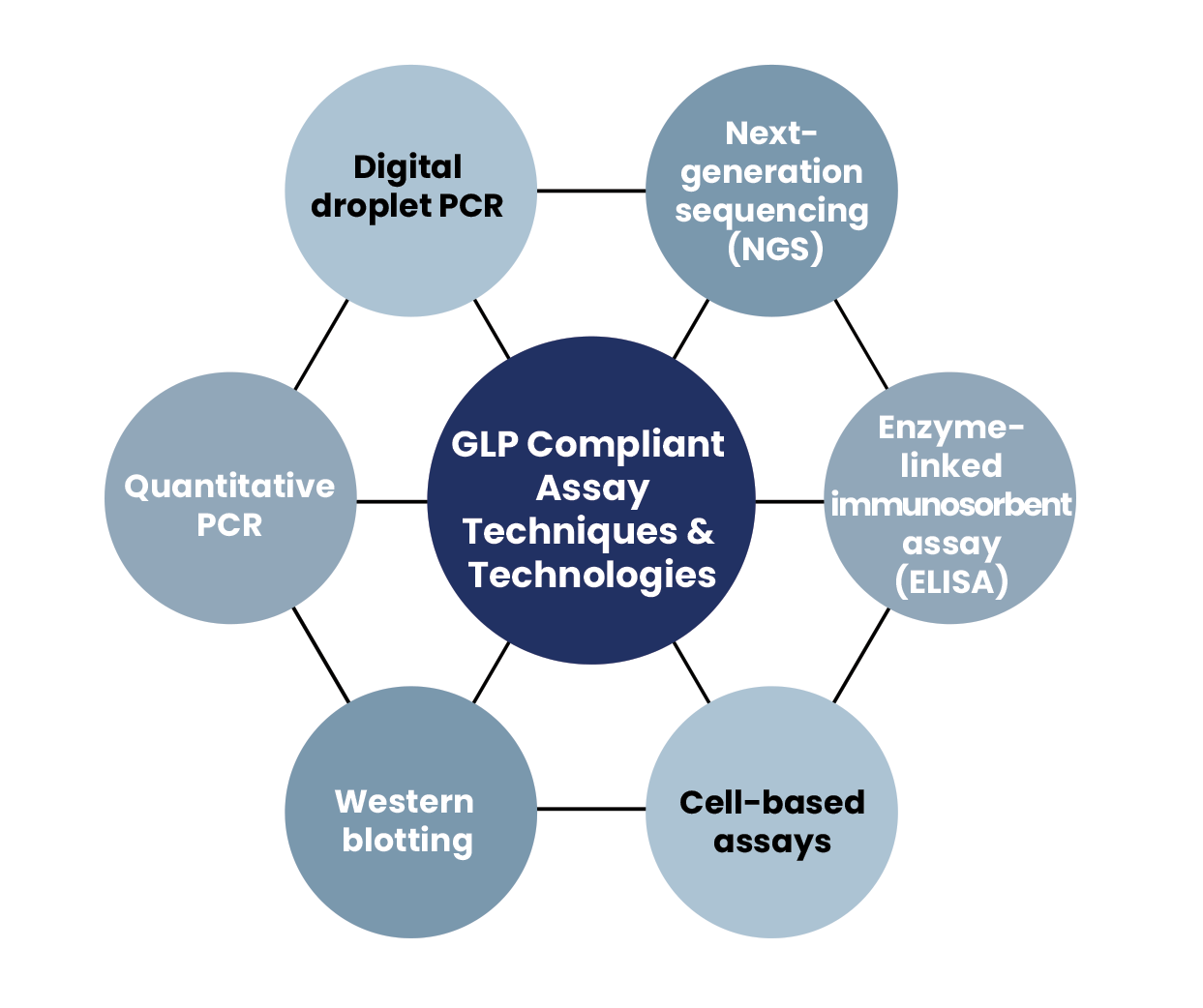
We offer tailored GLP-compliant assay solutions in several application areas, driving your discovery and development efforts forward with reliable, trusted results.
- DNA and RNA biodistribution studies
- Gene expression studies
- Protein studies with ELISA and western blot
- CRISPR on-/off-target evaluation
- Gene editing translocation evaluation
- Single-cell amplicon NGS assays
- NGS integration analysis for gene editing and gene therapy
- Vector copy number (VCN) analysis for inserted genes
- Colony forming unit assays (CFU)
- Viral titer determination
- Custom assay design and validation
Our Customers Say…
From the preliminary conclusion of this work, we are already pleased to thank you for your customer-oriented approach and proactive communication in the course of this study. We are happy to see the profound difference in business mindset between your organization and our previous vendor…
Project Manager, Belgium
Thank you so much for successfully completing this important project for us on time. We are very satisfied with the final report and thank you for taking the extra effort to customize the report format. We will definitely use your lab for future projects.
Program Manager, California
It has been a pleasure working with you and your team. The project was carried out to the highest standard and delivered in a timely manner. I will definitely recommend Avance Biosciences™’ services to colleagues and friends.
Senior Scientist, Singapore

