Ensure the Success of Your Biological Drug Discovery and Development
What We Do
Biopharmaceutical drug discovery and development requires a solid understanding of the therapeutic target, conformation and characterization of drug candidates, and customized solutions for pre-clinical and clinical testing.
We offer a broad range of assay development services, including assay design, assay validation, sample testing, and technology transfer under GLP compliance to support IND-enabling and clinical studies. With decades of experience helping gene and cell therapy companies, Avance Biosciences™ has the expertise and track record to assist you with your biological drug discovery and development.
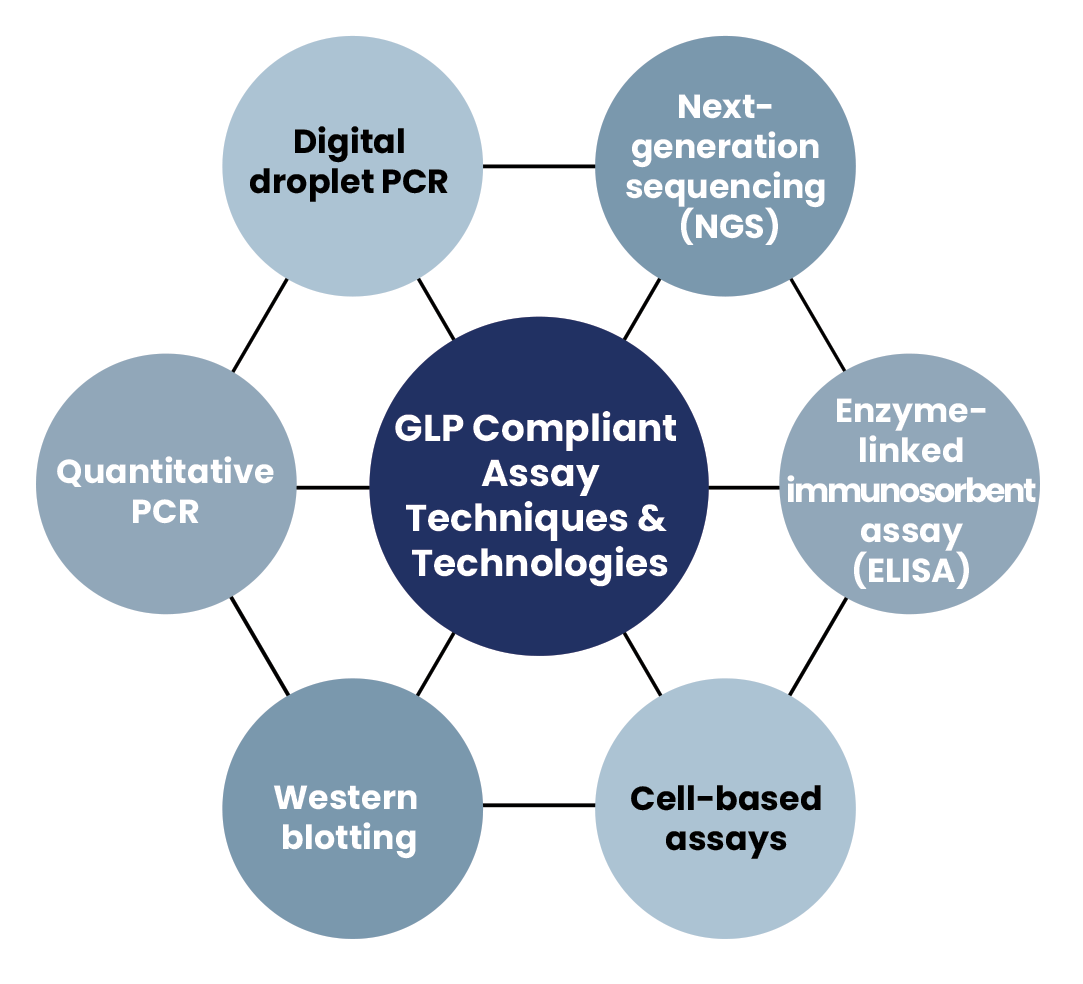
Our assays use the latest biological assay techniques and technologies, including:
Ensure the Success of Your Biological Drug Discovery and Development
Avance Biosciences™ is a world-leading CRO providing advanced and customized assay solutions to facilitate your biotherapeutic products’ research and development.
What We Do
Biopharmaceutical drug discovery and development requires a solid understanding of the therapeutic target, conformation and characterization of drug candidates, and customized solutions for pre-clinical and clinical testing.
We offer a broad range of assay development services, including assay design, assay validation, sample testing, and technology transfer under GLP compliance to support IND-enabling and clinical studies. With decades of experience helping gene and cell therapy companies, Avance Biosciences™ has the expertise and track record to assist you with your biological drug discovery and development.
Our assays use the latest biological assay techniques and technologies, including:

Applications
We offer tailored GLP-compliant assay solutions in several application areas, driving your discovery and development efforts forward with reliable, trusted results.
DNA and RNA biodistribution studies
Gene expression studies
Protein studies with ELISA and western blot
CRISPR on-/off-target evaluation
Gene editing translocation evaluation
Single-cell amplicon NGS assays
NGS integration analysis for gene editing and gene therapy
Vector copy number (VCN) analysis for inserted genes
Colony forming unit assays (CFU)
Viral titer determination
Custom assay design and validation

Our Approach To Your Project
We’re here to advance your biological discovery and development. We do it with solid science, strict regulatory compliance, and an experienced team to streamline the process.
Our Approach To Your Project
We’re here to advance your biological discovery and development. We do it with solid science, strict regulatory compliance, and an experienced team to streamline the process.
Project Management
Collaboration with you to develop and qualify assays, and perform sample testing
Dedicated project manager, senior scientist, and study director to lead your project
Regularly scheduled project check-in meetings to review data and discuss next steps
Flexible structure to accommodate changing requirements
Sound scientific approach and methods
Assay Development
Robust assay design
Specificity, sensitivity, accuracy, and precision determination
Development of appropriate control and reference samples
Advanced development and pre-qualification of all parameters associated with qualification and validation to fully characterize assay performance
Qualification and Validation
Follow FDA/ICH guidelines for assay qualification and validation
Parameters include specificity, sensitivity, precision/accuracy, robustness, extraction efficiency, and more
Mutually agreed on pass/fail criteria determined in advanced development
QA reviews all data, test plans, and final report
Ability to validate NGS data analysis pipeline



Project Management
Collaboration with you to develop and qualify assays, and perform sample testing
Dedicated project manager, senior scientist, and study director to lead your project
Regularly scheduled project check-in meetings to review data and discuss next steps
Flexible structure to accommodate changing requirements
Sound scientific approach and methods
Assay Development
Robust assay design
Specificity, sensitivity, accuracy, and precision determination
Development of appropriate control and reference samples
Advanced development and pre-qualification of all parameters associated with qualification and validation to fully characterize assay performance
Qualification and Validation
Follow FDA/ICH guidelines for assay qualification and validation
Parameters include specificity, sensitivity, precision/accuracy, robustness, extraction efficiency, and more
Mutually agreed on pass/fail criteria determined in advanced development
QA reviews all data, test plans, and final report
Ability to validate NGS data analysis pipeline
Who We Are
A world leading CRO providing GMP/GLP compliant biological testing solutions supporting the biological drug development pipeline from discovery through manufacturing. Our team has decades of experience in supporting biological drug development and manufacturing. With our technical expertise, focus on a strong adherence to regulatory guidelines, and dedication to exceeding our client’s expectations, we provide creative solutions to assure your drug development success.
Meet With Our Experts Today
We are a team of dedicated scientists, and quality control professionals focused on meeting your needs and completing your challenging drug development and manufacturing projects. By partnering with us, you’ll benefit from collaboration with a scientific team with decades of experience designing, validating, and executing biological assays and tests for regulatory submission. When you partner with Avance Biosciences™, you gain a CRO partner that is creative, collaborative, and dedicated to sound science with a focus on your specific regulatory requirements.
Our Customers Say…
Avance Biosciences™ (AB) has delivered the Sequencing data in a record time. Very impressive! We have benefited from AB’s hard work and are very grateful for that. AB team should feel proud of themselves. We thank AB team for their support and contributions, much appreciated.
Head of Quality Control, Microbial Manufacturing Service, California
Your team met the timelines we needed and did exceptional technical work. I will definitely make my management aware that Avance is a very capable partner for our bioanalytical needs.
Bioanalytical Senior Project Manager, California
My experience with Avance has been nothing but positive… I will keep sending work your way as I appreciate the attention your team provides and Avance’s creative technical team. See you soon.
Director Individualized Medicines, California
Avance has been the ideal partner in helping Mission Bio and our client,… be the first in the world to GMP qualify our single cell % transduction assay for a lentiviral gene therapy product. Excellent execution on the part of the Avance team. We look forward to more collaborations in the future.
Senior Director, Cell & Gene Therapy Business, California
From the preliminary conclusion of this work, we are already pleased to thank you for your customer-oriented approach and proactive communication in the course of this study. We are happy to see the profound difference in business mindset between your organization and our previous vendor…
Project Manager, Belgium
Thank you so much for successfully completing this important project for us on time. We are very satisfied with the final report and thank you for taking the extra effort to customize the report format. We will definitely use your lab for future projects.
Program Manager, California
It has been a pleasure working with you and your team. The project was carried out to the highest standard and delivered in a timely manner. I will definitely recommend Avance Biosciences™’ services to colleagues and friends.
Senior Scientist, Singapore

