Weill Cornell study demonstrates safety of intravenous administration of an AAV8 gene therapy
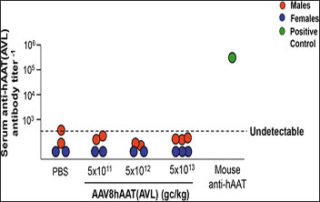
webmaster2025-10-06T21:50:14+00:00Researchers report on the safety of a gene therapy to treat the common autosomal recessive hereditary disorder alpha 1-antitrypsin (AAT) deficiency in a new article in the peer-reviewed journal Human Gene Therapy. In ATT deficiency, neutrophil proteases destroy the lung parenchyma, the portion of the lungs...