NGS Amplicon Sequencing Services
NGS amplicon sequencing is essential for gene editing, enabling precise on- and off-target analysis and characterization of indels and mutations. It provides high-resolution data on specific DNA regions, ensuring the accuracy and efficiency of tools like CRISPR, prime editing, and zinc-finger nucleases. This method helps ensure the safety and efficacy of gene and cell therapies by detecting unintended edits and optimizing therapeutic outcomes.
Our Expertise
Avance Biosciences added the Illumina MiSeq platform to our GMP services lab in 2013. Since this time, we have successfully completed numerous GMP grade NGS On/Off target analysis in support of our customers edited cell-based drug products. With our unparalleled understanding of Illumina NGS chemistry and our innovative solutions for addressing FDA Part 11 compliance, Avance Biosciences stands as a true leader in the GMP and GLP applications of NGS technology.
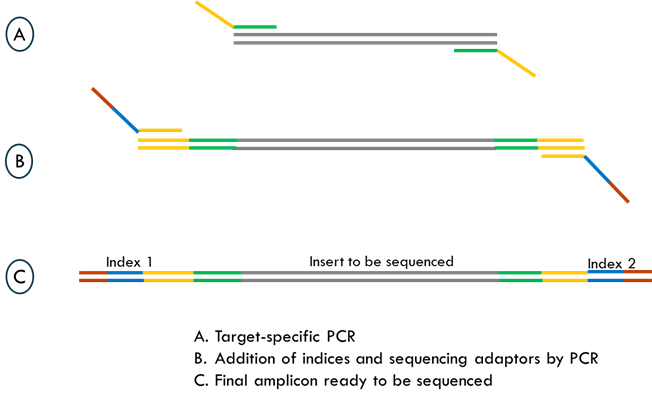
Workflow
Case Studies
- In 2013, we established a MiSeq NGS amplicon sequencing assay in compliance with GMP to support a major pharmaceutical client in validating a companion diagnostic real-time PCR assay. To our knowledge, this was the first instance of an NGS instrument being implemented in a GMP environment.
- In 2017, we supported a CRISPR-mediated cell therapy biodistribution study by performing NGS amplicon sequencing on animal tissues that tested positive with real-time PCR. The amplicon sequencing method was validated according to GLP regulations. This therapy later became the first-ever CRISPR-mediated cell therapy approved by the FDA in 2023.
- Since 2017, we have developed numerous quantitative NGS amplicon sequencing assays to support animal studies and clinical trials. Each assay is uniquely designed and validated according to FDA requirements for bioanalytical method validation. Our NGS analysis pipelines are also validated, with a proprietary server environment ensuring long-term stability of pipeline performance and maintaining an audit trail.
- Since 2019, we have designed and validated numerous rhAMPSeq assays to evaluate on- and off-target effects, as well as the percentage of indels and mutations in cells edited with gene editing tools such as CRISPR, prime editing, and zinc-finger nucleases.
- In 2021, we collaborated with Mission Bio to validate a Tapestri-based single-cell sequencing assay to support a client’s hematopoietic stem cell (HSC) therapy. Multiplex amplicon sequencing was used to test cells with positive gene editing, determining transduction efficiency at the single-cell level.