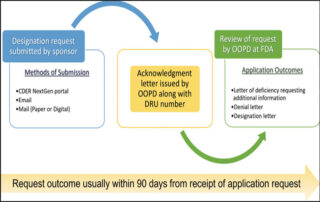
Enhancing Safety and Efficacy – Biodistribution Services for Advanced Cell and Gene Therapies
webmaster2023-07-11T18:18:43+00:00As the field of advanced cell and gene therapies continues to grow exponentially, ensuring their safety and efficacy becomes paramount. Biodistribution studies, which evaluate the distribution of viral vectors and transgenes in the body, play a crucial role in pre-clinical pharmacokinetics and toxicity assessments...