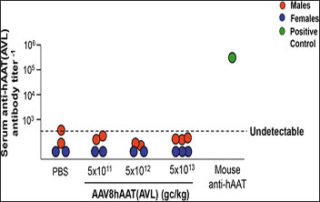
NICE recommends life-changing gene therapy for children with ultra-rare genetic disorder
webmaster2025-10-06T21:50:13+00:00A single dose gene therapy designed to correct the underlying genetic defect, eladocagene exuparvovec (also called Upstaza and made by PTC Therapeutics), is infused directly into the brain through a minimally invasive procedure – the first gene therapy to be administered in this way...