Technote: Phage Testing of Bacterial Cultures
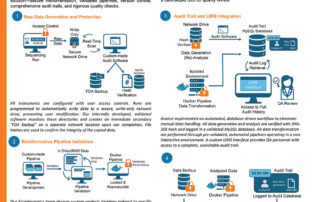
webmaster2025-12-23T18:19:16+00:00Download our whitepaper on “Phage Testing of Bacterial Cultures” to understand the critical importance of mitigating phage contamination in biotech and pharmaceutical industries. Learn about the lytic and lysogenic life cycles of phages, their role in bacterial biology, and the challenges they pose to the biotech and pharmaceutical industries due to contamination. Phage presence in cultures can significantly skew results, posing challenges in bacterial identification and isolation...