News
Your source for the latest updates, breakthroughs, and insights in drug development and manufacturing. Explore our featured stories, press releases, and event announcements to stay informed about our innovations and contributions to drug development and manufacturing advancements.
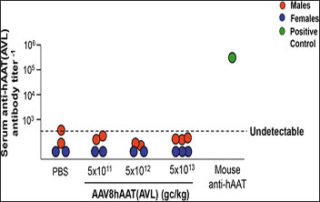
Weill Cornell study demonstrates safety of intravenous administration of an AAV8 gene therapy
Researchers report on the safety of a gene therapy to treat the common autosomal recessive hereditary disorder alpha 1-antitrypsin (AAT) deficiency in a new article in the peer-reviewed journal Human Gene Therapy. In ATT deficiency, neutrophil proteases destroy the lung parenchyma, the portion of the lungs...
mRNA Analytical Development Summit – 2023
With several years of experience supporting a world-renowned mRNA COVID-19 vaccine company with raw material and drug substance release, Avance Biosciences™ is well-positioned to provide industry-leading assay design, validation, and sample testing services that advance our clients’ mRNA therapeutic...
FDA clears Sana Biotechnology’s CAR T Therapy IND application for patients with B-cell malignancies
The goal of the hypoimmune platform is to overcome the immunologic rejection of allogeneic cells, which if true for SC291 may result in longer CAR T cell persistence and a higher rate of durable complete responses for patients with B-cell lymphomas or leukemias. The hypoimmune platform includes disruption of major...
Scientists develop novel mRNA delivery method using extracellular vesicles
In the study, published in Nature Biomedical Engineering, researchers from MD Anderson Cancer Center use EV-encapsulated mRNA to initiate and sustain collagen production for several months in the cells of photoaged skin in laboratory models. It is the first therapy to demonstrate this ability and represents a proof-of-concept for deploying the EV mRNA therapy...
ASGCT Members Provide Recommendations During FDA Liaison Meeting
ASGCT held its fifth annual liaison meeting with FDA CBER’s Office of Tissues and Advanced Therapies (OTAT) on Nov. 14, 2022. A group of Society leaders and members gave two presentations to FDA on significant topics in the field, followed by a presentation from FDA. Dr. Keith Wonnacott, ASGCT’s Regulatory Affairs Committee Chair, chaired the meeting and moderated discussion...
CG Oncology advancing clinical-stage urologic oncology immunotherapy pipeline
Company files its first Investigational New Drug application for its lead program KYV-101, a novel fully human CD19 CAR T-cell therapy, for the treatment of lupus nephritis. Kyverna’s therapeutic platform combines advanced T-cell engineering and synthetic biology technologies to suppress and eliminate...