FDA approves the first gene therapy for people with beta-thalassemia who require regular red blood cell transfusions
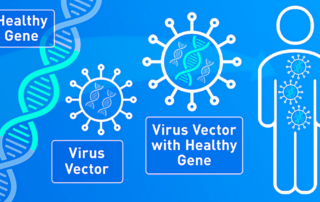
webmaster2025-10-06T21:50:15+00:00bluebird bio has announced the the U.S. Food and Drug Administration (FDA) has approved ZYNTEGLO® (betibeglogene autotemcel), also known as beti-cel, a one-time gene therapy custom-designed to treat the underlying genetic cause of beta‑thalassemia in adult and pediatric patients who require regular red blood cell (RBC) transfusions. “The FDA approval of ZYNTEGLO offers people with [...]