GUIDE-Seq/iGuide for CRISPR On/Off Target Analysis
To assess the risks of new human gene editing products, the FDA recommends using multiple orthogonal methods (e.g., in silico, biochemical, cellular-based assays) to identify putative off-target editing sites. GUIDE-Seq/iGuide is one of the techniques that is frequently employed by our gene and cell therapy clients to analyze the on- and off-target effects of CRISPR-Cas9 gene editing. It provides valuable information about the specificity and accuracy of the CRISPR system, serving as a valuable aid in the selection of optimal gRNA, nucleases, and other CRISPR conditions, while also enabling the assessment of the safety of gene-editing-based therapeutics.
Our Expertise
Avance Biosciences, a licensed Guide-Seq service provider, boasts extensive experience in assisting leading gene and cell therapy clients with the comprehensive characterization of their CRISPR-edited in vivo and ex vivo therapeutics. Our successful collaborations with prominent pharmaceutical and biopharmaceutical companies have contributed to the study of on/off-target profiles for their respective gene and cell therapy INDs. Reach out to us today to explore our on/off-target gene editing services and leverage our expertise in navigating the dynamic CRISPR gene editing domain.
Workflow
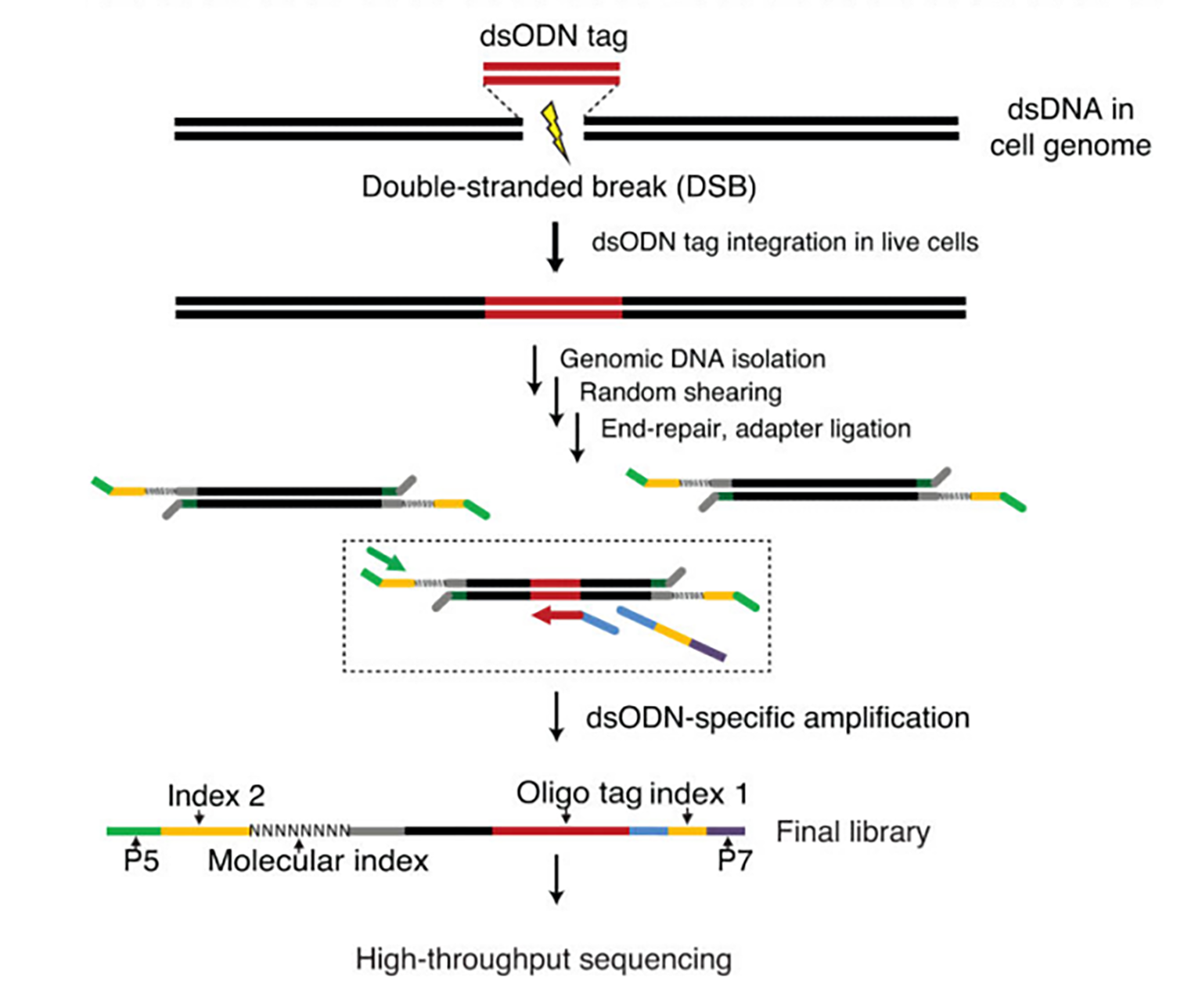
The GUIDE-Seq workflow involves integrating a double-stranded oligodeoxynucleotide (dsODN) at double-stranded breaks, followed by targeted amplification and sequencing to precisely identify and map off-target cleavage sites in CRISPR-edited genomes.
Critical Success Factors
Indicator Cell Selection
The choice of an indicator cell type is critical in the design of an effective Guide-Seq/iGuide experiment. It is crucial that the indicator cell line closely mirrors the anticipated gene editing product. Cell lines displaying a strong DNA repair response, such as HEK293 and CD4 T-cells, are advisable for heightened sensitivity in detecting off-target effects.
Control Design
To reduce sequencing background noise during the data analysis phase, it is crucial to incorporate suitable positive and negative controls in GUIDE-seq or iGUIDE experiments. This commonly entails sequencing gene-edited samples alongside both naïve cells and positive controls.
Technical Information
Download our whitepaper on “Phage Testing of Bacterial Cultures” to understand the critical importance of mitigating phage contamination in biotech and pharmaceutical industries. Learn about the lytic and lysogenic life cycles of phages, their role in bacterial biology, and the challenges they pose to the biotech and pharmaceutical industries due to contamination. Phage presence in cultures can significantly skew results, posing challenges in bacterial identification and isolation...
Genome editing offers unprecedented precision, but off-target effects remain a key safety concern. GUIDE-Seq provides a high-throughput, genome-wide method to map CRISPR-Cas activity in living cells, enabling researchers to assess specificity and ensure safer gene-editing therapies...