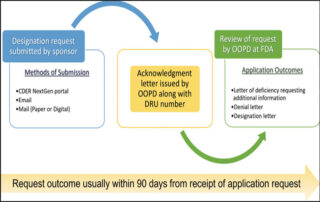
2seventy bio presents broad range of new data highlighting novel approaches across its cell therapies portfolio
webmaster2025-06-24T13:49:48+00:002seventy bio announced new data featuring novel approaches combining the company’s platform CAR T cell and T cell receptor technology and unique cell therapy engineering capabilities to potentially enhance treatment potency in a range of cancers...